
For half a century, researchers have pondered how gene drives found in nature could be repurposed to combat mosquito-borne illnesses. In 1960, the biologists George Craig, William Hickey, and Robert Vandehey proposed fighting illnesses carried by the Aedes aegypti mosquito (which include dengue, yellow fever, and now Zika) using a selfish genetic element that biases offspring to tilt more heavily toward males than females; female mosquitoes are the only ones that bite, so a male-biased population would mean fewer mosquitoes that can spread disease. In 1968, Chris Curtis, a medical entomologist, developed a model showing how the use of such a “desirable gene” could spread beneficial traits throughout an insect population — by, say, “mak[ing] mosquitoes non-infectible by pathogens.”
As genetic engineering became more and more practical, biologists like Margaret Kidwell and José Ribeiro in 1992 and Austin Burt in 2003 began to map out how exactly to make the 1960s biologists’ plans a reality — to either make mosquitoes disease-resistant (Kidwell and Ribeiro) or crash mosquito populations and render mosquito-borne diseases impossible to spread (Burt’s primary focus). But gene editing was still hard.
That all changed in 2012, when Doudna and Charpentier showed that CRISPR could make gene editing easy and reliable. It’s cheaper, faster, and less error-prone than any gene editing technology that came before it. Esvelt realized exactly what this meant for gene drives. In 2014, as a fellow at Harvard’s Wyss Institute, he and professors George Church and Flaminia Catteruccia and graduate researcher Andrea Smidler published a paper explaining how CRISPR enabled the creation of the kinds of gene drives prophesied for more than five decades. It’s hard to imagine a gene more selfish than one that uses CRISPR to insert itself into every egg or sperm it can.
CRISPR would “enable scientists to construct efficient … gene drives not only in mosquitoes, but in many other species,” they wrote. Insects like the Western corn rootworm and plants like the horseweed and pigweed that disrupt farming could be rendered nonresistant to pesticides and herbicides, or changed so they no longer like the taste of the crops they prey on. Invasive species like Asian carp could be eradicated from habitats they’ve invaded. Vulnerable or endangered species could be engineered to be more fertile.
One approach: using gene drives to make mosquitoes immune to malaria
Not even two years after that paper was published, a team of scientists at UC San Diego and UC Irvine announced that they had made a CRISPR gene drive for the Anopheles stephensi mosquito, which is a major vector of malaria in India and South Asia. The gene drive spread genes that offered resistance to the malaria parasite, as well as changes to eye color to confirm the changes had been made.
The team began with just two edited males, designated mosquitoes 10.1 and 10.2, into which the drive was inserted. After two generations of cross-breeding with hundreds of wild-type mosquitoes — and in mosquitoes, two generations can pass in less than a month — they produced 3,894 third-generation mosquitoes, of which 3,869 (99.5 percent) had the resistance gene. Just two mosquitoes were able to spread the trait to thousands of progeny — and malaria resistance along with it.
Ethan Bier and his then-grad student Valentino Gantz, who were co-authors on that paper, came to gene drives through not mosquitoes but flies, and basically by accident. They knew about a gene that, in one species of fly (the Drosophila melanogaster or fruit fly, and one of the most-used animals in genetics experiments), caused one vein in the fly’s wing to disappear. Gantz wanted to see if that gene, added to another fly, the Megaselia abdita or “humpback” fly (which in a talk he described as the fly “that infests your trash at home”), would cause two veins to disappear.
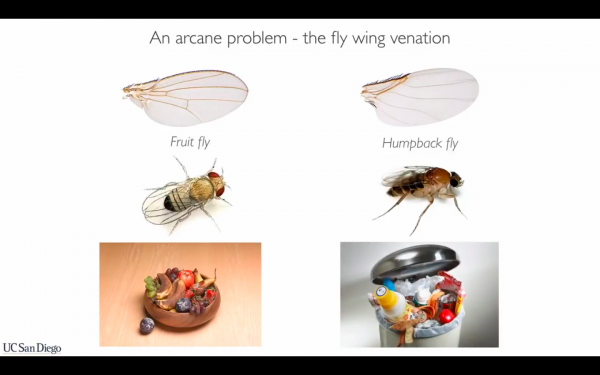
To make the edit they wanted, they wound up deploying a CRISPR gene drive. They started with Drosophila and added a gene to change body color from the normal brown to yellow. After a couple of generations, they were looking at vials full of yellow flies — 95 to 100 percent, after breeding nine mutated yellow flies with hundreds of wild flies.
“We were not very hopeful. We’re like, ‘Well, it’s just not going to work,’” Gantz told my colleague Joss Fong. “We were amazed when we actually saw the results and all the flies were yellow, which is something that shouldn’t happen, and that all the progeny was yellow too.”
Six days after they submitted their findings, they contacted Anthony James, a geneticist at UC Irvine who studies mosquitoes and malaria. His life’s work, since 1986, has been spent figuring out which genes can make mosquitoes resistant to the parasite. “The objective of our work was to build genes that when placed into the mosquitoes would confer a resistance phenotype — that is, the mosquitoes will not be able to transmit,” he told Fong. “And once we built those genes, then we needed a way to disperse them rapidly through mosquito populations.”
Bier and Gantz realized that with their fly experiment, they had developed a way to disperse the changes James had developed rapidly. “It was clear to me at that time that they had a gene drive system,” James said. “We were riding a bicycle and then along came this fast train, and so we switched the bicycle for the train.”
The result of their collaboration was the creation in 2015 of the Anopheles stephensi gene drive. It’s more of a proof of concept than an actually deployable drive; if released into the wild in India, it’s likely the malaria parasite would develop resistance to the genetic change (though not before spreading widely and perhaps into Pakistan, with all the political dangers that entails).
But the drive Bier, Gantz, James, and the rest of the team developed nonetheless serves as a model for an approach to malaria gene drives sometimes known as “alleviation drives,” which are meant to spread traits to mosquitoes meant to render them invulnerable to malaria infection. I’ve heard some scientists call this the “California school” of malaria gene drives, given where Bier, Gantz, and James all work, along with other SoCal mosquito/gene drive researchers like UCSD’s Omar Akbari or Caltech’s Bruce Hay.
Another option: reducing mosquito populations, not immunizing them
Some 5,500 miles away, you’ll find the “London school” of malaria gene drives, spearheaded by Imperial College London’s Austin Burt, whose 2003 paper on gene drives remains a crucial model for experiments even today, and Andrea Crisanti, who works more directly with mosquitos. Target Malaria, the group closer than any other to a field deployment of a mosquito gene drive, is hoping to eventually deploy in Africa variants of the mosquitoes Crisanti designs in his lab in London.
The London school’s focus is not on creating mosquitoes that are invulnerable to malaria, the way the California school’s is. The London school is all about reducing the number of malaria-transmitting mosquitoes out there. That means spreading genes that, for example, bias sex ratios to create more males, leaving fewer female mosquitoes to bite people and causing a population crash, or genes that lead to female infertility, which have a similar outcome. Gene drives that do this are called suppression drives because they suppress the population. And they’re making big progress; a paper released by the London team in September 2018 found that a suppression gene drive they designed reached 100 percent prevalence among mosquitos in a lab after 7-11 generations.
This approach might sound disturbing from an ecological perspective, but mosquitoes don’t make up a significant portion of any known predator’s diet; there’s just not a lot of meat there. “So far there’s no evidence really that seems to show that Anopheles gambiae is a key species in the ecosystem,” Jonathan Kayondo, a senior research officer at the Uganda Virus Research Institute and a member of Target Malaria’s scientific team, told Fong. “There’s nothing that exclusively feeds on it. So I’m finding it hard to see how that would collapse the ecosystem, because that’s the fear most people have.”
I want to be really, really clear here: No one is talking about killing all mosquitoes. There are some 3,500 species of mosquitoes in the world, and only a small fraction of them transmit any diseases, let alone malaria. Target Malaria is currently targeting only three species: Anopheles gambiae, Anopheles coluzzii, and Anopheles arabiensis.
Less than two months after the Bier/Gantz/James paper describing an alteration drive for Indian malarial mosquitoes was published, a team including Crisanti and Burt published their own paper explaining that they had created a gene drive for A. gambiae (which is technically a species complex rather than a species) that spread genes causing female sterility. A. gambiae and a couple of closely related variants are by far the dominant malaria vectors in Africa, where nearly 90 percent of malaria deaths occur. When it comes to fighting malaria, it’s a more important species than the A. stephensi that the California researchers focused on.
As of late 2015, California and London schools had both created the organisms they hope, with just a bit of tweaking, can save as many as 720,000 lives every year.

Who gets to regulate an insect that changes an entire ecosystem?
More scientific work needs to be done before gene drives are ready for release in the wild. But the hardest part of the development phase is over. The problem of malaria gene drives is rapidly becoming a problem of politics and governance more than it is a problem of biology.
Robert Friedman, the vice president for policy at the J. Craig Venter Institute and a prominent voice on questions of how to regulate gene drives, says the timeline for gene drive release will be “driven more on the regulatory side than the science side.”
“There are no regulators that have handled this before,” Kayondo, the Target Malaria researcher, told Fong. “Not just in Africa, but anywhere.”
And how, really, do you go about regulating something like this — especially when the technologies being developed are made by Western scientists, to be deployed for the benefit of African people in African communities?
“With malaria, I think, who are the people mostly affected and most affected by decisions made about gene drives?” Keisha Ray, a professor of philosophy and bioethicist at Texas State University, says. “It’s not going to be the wealthy people in wealthy countries. It’s going to be people of color; it’s going to be poor people.”
Groups working on gene drives, and on their possible deployment, are immensely sensitive to that dynamic. They insist, usually without prompting, that the single most important thing that scientists and NGOs have to do is give African communities the ultimate say in whether or not to use a technology that’s meant to help them.
Multiple people brought up the African Union’s New Partnership for Africa’s Development (NEPAD) as an organ that could ultimately sanction the release of a gene drive on the continent. Target Malaria scientists, like Kayondo in Uganda, have already consulted with NEPAD about gene drives. The Open Philanthropy Project, a charitable group in the US that Facebook billionaire Dustin Moskovitz and others have tasked with giving away their fortunes, gave NEPAD more than $2 million to work on gene drive issues.
But we’re a long way away from NEPAD making that decision. There’s considerable work left to do in harmonizing countries’ regulatory systems and achieving buy-in from all member countries that could be affected.
Target Malaria is starting out, very cautiously, by seeking regulatory approval to release male mosquitoes that have been genetically engineered to be sterile in Burkina Faso; the government approved the request in September, 2018. These aren’t gene drive mosquitoes; gene drive mosquitoes aren’t sterile and spread their genetic change throughout a whole population, whereas sterile males won’t pass on any genes that affect the broader mosquito population.
Just dumping a bunch of sterile males isn’t likely to do much to fight malaria, by Target Malaria’s own admission; you’d need to release millions of sterile mosquitoes in dozens of villages until you’d start inconveniencing the mosquitoes. But the group is releasing sterile males not to fight malaria but as a first step, to show local communities what their work is like, that they can be trusted, and that genetically modified mosquitoes aren’t necessarily something to be feared.
The project requires consulting extensively with people living in and near the villages where the mosquitoes would be released, making contact with community leaders, and ultimately getting Burkina Faso’s national regulatory body for medical and scientific trials to give its go-ahead. (The mosquitoes are currently in Burkina Faso, waiting for government approval to be released.)
Target Malaria is hoping to eventually work up to gene drive mosquitoes, starting with the sterile males, then move to what’s called an “X-shredder,” a genetic modification that rips up the X chromosome of male mosquitoes so that they only pass on Y chromosomes and have almost exclusively male offspring. The population of females would take a hit, but gradually the population would return to normal as the generation produced by the X-shredder died off and its offspring, with a normal mix of male and female, took over.
Only after trying an X-shredder, which would reduce malaria transmission but not eliminate it, would the group consider releasing a gene drive. And once it does, it’s hard to know where it will stop. A drive released in Burkina Faso could spread throughout the mosquito population and affect mosquitoes as far away as South Africa. A Burkina Faso field trial absolutely will not remain a Burkina Faso field trial. A Burkina Faso field trial isn’t even a field trial at all. It could eventually result in continent-wide deployment of the technology.



The backlash against gene drives
The mere possibility of a live deployment of gene drives has some environmental watchdogs critical of biotech like the ETC Group and Friends of the Earth up in arms. In late 2016, activists unsuccessfully lobbied a meeting of the parties to the Convention on Biological Diversity, an international treaty covering biodiversity and related genetic issues, to impose a moratorium on gene drive field trials, as well as some lab experiments.
They raised concerns about militarization, noting that DARPA, the R&D arm of the Defense Department, has funded gene drive research. “There are many scenarios for potential weaponisation of gene drives as well as serious potential ramifications from unintended effects,” Jim Thomas, the research program director at the ETC Group, wrote at the Guardian. “Imagine for a moment that a hostile actor could crash the harvest of an island state by quietly introducing a gene drive or could insert a gene drive into a biting insect population to deliver toxins.”
The biosecurity experts I talked to are deeply skeptical of those nightmare scenarios. Sonia Ben Ouagrham-Gormley, a professor in the biodefense program at George Mason University, says she doubts gene drives will be militarily effective in targeting rival countries’ harvests. “Animals and plants that are raised for food are generally monitored, and a gene drive can be easily detected in the genome of the animal,” she explained. “Because of that regular monitoring, I don’t think gene drives would be a good tool for affecting a country via agriculture.”
Filippa Lentzos, a biosecurity expert and senior research fellow at King’s College London, agrees. Lentzos has studied gene editing labs with an eye to these kinds of dangers. Because gene drives affect only sexually reproducing species, they can’t be used to make bacteria or viruses more potent.
“Gene drive technology is biased toward defense,” Esvelt says. “It spreads slowly over generations, is unfailingly detectable if you look for it, and can be reliably countered by overwriting the unwanted changes. Anything slow, obvious, and easily blocked isn’t much of a physical threat.”
The threats he and bioweapons experts like Ouagrham-Gormley and Lentzos worry about more are social. What happens if one of the few thousand fruit fly biologists around the world decides to act unilaterally and throws international talks on the matter into chaos? What if a grad student creates a gene drive that can’t reliably hurt people but can reliably terrify them?
Beyond the military question, opponents of gene drives just don’t think it’s acceptable to release an organism with this much potential to spread. “There isn’t a safe way to experiment with technologies like these in the wild,” Dana Perls, a senior food and technology campaigner at Friends of the Earth, told Fong. “Until there are robust, international, sensible regulations and oversight to determine if gene drives are even possible to use safely, we need to hit the pause button on gene drive development everywhere.”
But that raises an even harder question: Is it sometimes necessary, even morally required, to release into the wild a technology that’s never been tested in the wild, because the cost of not doing so is just too high?
Recall that between 438,000 and 720,000 people die of malaria every year. So starting from the moment we have deployable gene drive technologies that could wipe out the disease, every day we wait kills between 1,200 and 2,000 people.
That doesn’t mean that a wily grad student should steal either the London or California mosquitoes and run out to release them immediately. The political costs of doing that could be immense. Friedman notes that AquAdvantage, a fast-growing genetically modified salmon, was first developed in 1989, and its creators are still fighting for the right to sell it in the US. Can you imagine the kind of political backlash that would follow the unsanctioned release of gene drive mosquitoes?
“Gene therapy was set back due to one tragic death. There was over a decade’s worth of delay to the field,” Esvelt notes. He was referencing Jesse Gelsinger, a healthy 18-year-old who died in 1999 when a gene therapy trial for which he’d volunteered at UPenn went awry. “How much worse would it be with gene drive, where you have it spread from continent to continent?” (Target Malaria’s mosquitoes wouldn’t spread beyond Africa. But drives targeting other species, in theory, could.)
An unsanctioned release wouldn’t succeed in eradicating malaria, he argues. Resistance would appear, and without a global effort monitoring the disease, it would be impossible to see where to combat it. Malaria could grow deadlier, as people who’d been infected in the past and built up resistance would be vulnerable again. And if the mosquitoes made it to Europe, the continent could impose economically harmful trade sanctions on African nations in the name of keeping them out.
For that reason, Esvelt argues that for most everything except malaria in Africa, we should be thinking much more about “self-exhausting” gene drives than ones that propagate without end. “Daisy drives,” an idea Esvelt and his lab have pioneered, would work for several generations but then stop spreading, limiting the extent to which they could dominate a species. “Threshold drives” are designed to spread in environments where they’re common but lose power as they become rarer relative to organisms that don’t have the drive mutation.
He concedes only a self-propagating drive would be sufficient to wipe out malaria in Africa (other techniques might be enough to do so in Asia). It might be necessary for a few other cases too, like schistosomiasis (a disease caused by parasitic worms that’s been linked to lower earnings and much worse quality of life) or New World screwworm, an agricultural predator that’s been wiped out in North America but still causes extraordinary pain and death among mammals raised for food in South America, literally eating them alive from the inside.
But precisely because it might be necessary for those limited cases, Esvelt argues that overreach that could put those efforts in danger is one of the worst mistakes scientists could make. Instead, he wants to use gene drive development as a test case for what he calls “responsive science”: science that places a premium on openness, announcing experiments before they happen and sharing data and research methods freely to receive maximum feedback from both community members and other scientists, and giving affected people the final word in whether to go ahead with a project.
“The most important ecosystem to be engineered is the scientific enterprise,” he says. “That’s because the future of our civilization will primarily be determined by the technologies we develop and the wisdom with which we choose to deploy them or refrain.”

What does an ethical approach to editing ecosystems look like?
Esvelt’s vision of responsive science isn’t purely theoretical. He’s already testing it in an environment radically different from the Burkina Faso villages where Target Malaria works: the posh island getaways of Nantucket and Martha’s Vineyard.
He and colleagues have proposed fighting Lyme disease by inundating the islands with genetically engineered mice that cannot spread the disease between ticks. He would not use a self-propagating gene drive — it would be unnecessary for the task at hand and an irresponsible use of a fickle technology. And at the end of the day, it’s not what the people there want.
The islands have “each appointed a steering committee to govern the project,” he says. “It’s entirely dependent on what they say should happen. We provide the options in terms of what can be done, and then we’re the technical hands. That’s what our vision of responsive science is.”
And the scientists have learned things in the process. Esvelt recalled: “During a Nantucket town hall meeting, someone … said, ‘Oh wait a minute, if you’re immunizing the mice against both Lyme and ticks, what if the ticks bite the mouse and then fall off? That first year, a lot of those ticks would be infected. Does that mean they’re then going to bite people?’”
“I turned and looked at Sam Telford [an ecologist working on the project], and he looked at me, and I said, ‘You know, we never considered that. That’s exactly why we’re here.’”
Esvelt’s right-hand adviser in the process of building a more responsive science is Jeantine Lunshof, a bioethicist based in his lab at MIT who also works with George Church at Harvard. A philosopher by training, she spends her days now surrounded by scientists, prodding them to ask better questions, to think about the least ethically risky ways to do experiments, and to encourage them to engage with what the philosophical tradition says about their life’s work.
After Esvelt told me he identified as a consequentialist — someone who judges the morality of actions by the goodness or badness of their consequences — Lunshof added, with a hint of pride, that he used to identify as a utilitarian (where “goodness” is identified with pleasure and “badness” with pain) until she convinced him to use the less extreme-sounding label.
Talking about him and the project of responsive science, Lunshof evokes another tradition more strongly: virtue ethics, the notion, rooted in Aristotle’s work and continued by 20th-century philosophers like Elizabeth Anscombe, Philippa Foot, and Alasdair MacIntyre, that morality has more to do with cultivating virtuous traits, valuable ways of being a person, than evaluating one’s actions in isolation. She’s not a virtue ethicist herself, seeing it as particularly problematic when applied to medical situations (“What are patient rights worth, if they clash with the virtues pursued by the doctor?”), but Esvelt inspired talk of virtue nonetheless.
“Kevin is, I’d say, a virtue-driven scientist,” Lunshof says. “This is a virtuous endeavor.” That, she is clear to emphasize, does not immunize it from criticism. Virtuous people have done unvirtuous things since the beginning of time. But in the face of extraordinary uncertainty about this technology and its effects, it’s a somewhat refreshing and revealing way to think about the ethical problems it faces.
Are the people developing gene drives being virtuous? Are they being generous in sharing their data? Are they being courteous in talking to the people affected? Are they being humble in not promising too much? Are they being decisive in moving to act when action is needed?
From the outside, right now, it looks that way. People are being careful. They’re proceeding vigorously but not with reckless abandon or excessive hubris. They know the tremendous stakes of the work placed before them and seem to feel that weight too — and they impressed upon me that I, as someone writing about this, should feel that weight as well.
When I told Lunshof about Esvelt’s warning, that botching this article could kill thousands of children, she laughed and added, “Oh, don’t worry so much!”
Read more
For more on the basic science, see the explainers from my colleagues on the Vox science desk about both CRISPR in general and gene drives in particular.
I’m hardly the first journalist to notice that CRISPR gene drives are a huge, huge deal, and there are a number of excellent feature articles that anyone interested in the topic should check out. They include:
- Michael Specter’s “Rewriting the Code of Life” in the New Yorker, profiling Kevin Esvelt and his “responsive science” model of developing and promoting gene drives.
- Jerry Adler’s “Kill All the Mosquitoes?!” in Smithsonian Magazine, which follows Andrea Crisanti and focuses on the ethical problems posed by the technology.
- Antonio Regalado’s piece in Technology Review, which focuses on Crisanti’s lab at Imperial College London.
- Ike Swetlitz’s piece for STAT, which goes on the ground in Burkina Faso to where Target Malaria is hoping to test sterile male mosquitoes and work up to testing gene drives.
- Sharon Begley’s profile, also at STAT, of Jeantine Lunshof, the bioethicist working with Esvelt and George Church and ensuring that gene drive developers and other CRISPR pioneers take the ethical questions posed by their work seriously.
- Ed Yong’s “One Man’s Plan to Make Sure Gene Editing Doesn’t Go Haywire,” at the Atlantic, using Esvelt’s work in Nantucket and Martha’s Vineyard as a model for how to do community engagement on gene editing in public health.
- Daniel Engber’s “Let’s Kill All the Mosquitoes” at Slate, which argues that, well, we should kill all the mosquitoes. To be clear, Target Malaria is looking at only 3 species out of 3,500, and most mosquitoes can probably live, but someone had to go out there and make the case, and Engber did it very well. See also Melissa Cronin at Vice arguing that maybe let’s not do that immediately.
- While not about gene drives, Sonia Shah’s The Fever is an incredible book that anyone interested in malaria should check out.
Vox senior editorial producer Joss Fong conducted interviews and contributed reporting for this story.
Sourse: breakingnews.ie
0.00 (0%) 0 votes